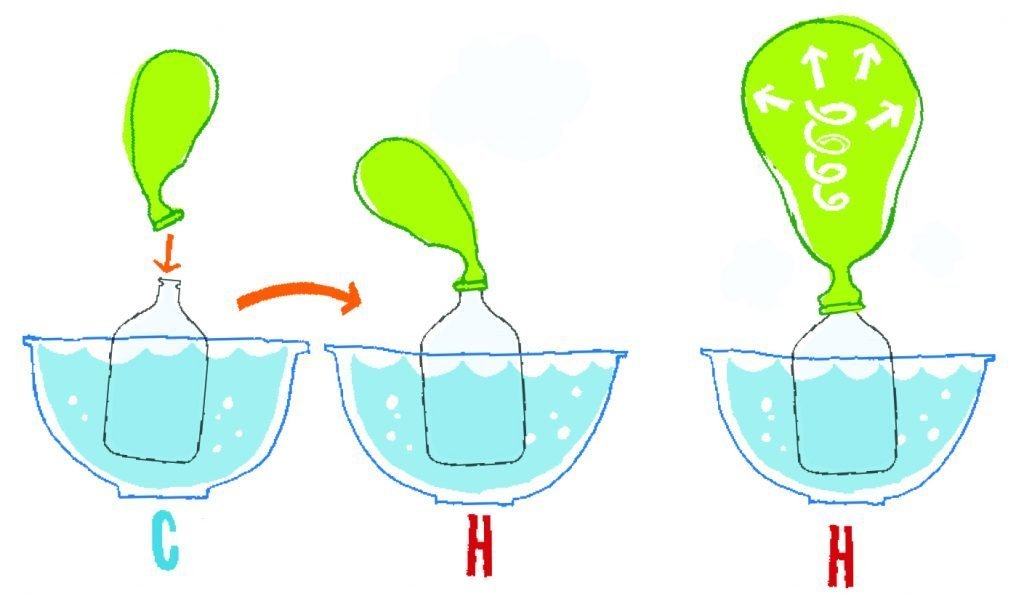
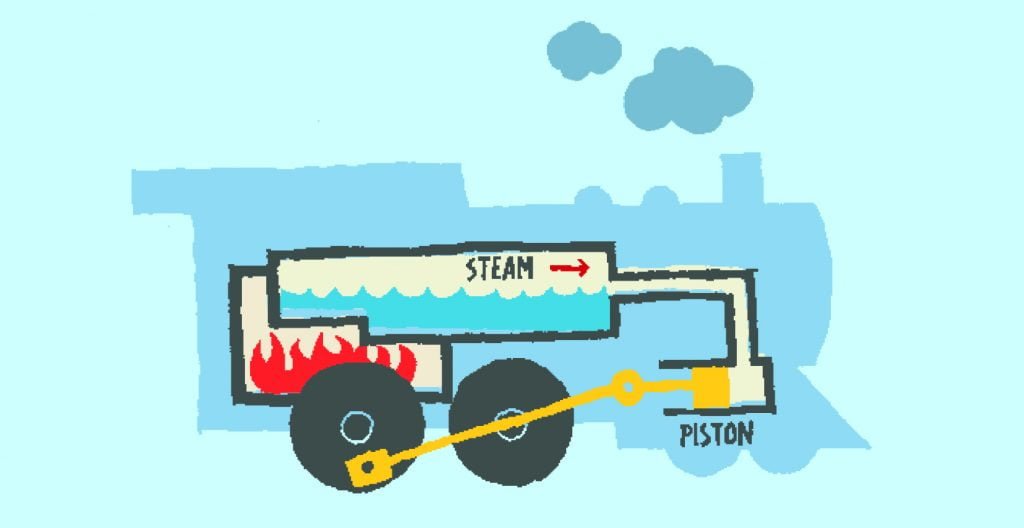
Find out what happens when gases are heated up or cooled down. The gas expands when it is heated. The rule is that if the gas’s pressure remains constant, the volume of the gas will increase as the temperature increases. So if the temperature increases, the gas takes up more space. This is known as Charles’ Law. The principle was first formulated by the French physicist Jacques Alexandre Cesar Charles in 1787. Today, steam engines heat up air and allow it to expand in cylinders to drive wheels.
Ingredients:
- Two bowls
- Cold water, hot water (with adult supervision)
- A sturdy plastic bottle
- A balloon
STEP 1
Fill two bowls – one with cold water the other with hot water.
STEP 2
Put the bottle into cold water.
STEP 3
Fit a balloon to the neck of the bottle.
STEP 4
Now place the bottle into the hot water.
STEP 5
Watch the balloon expand.