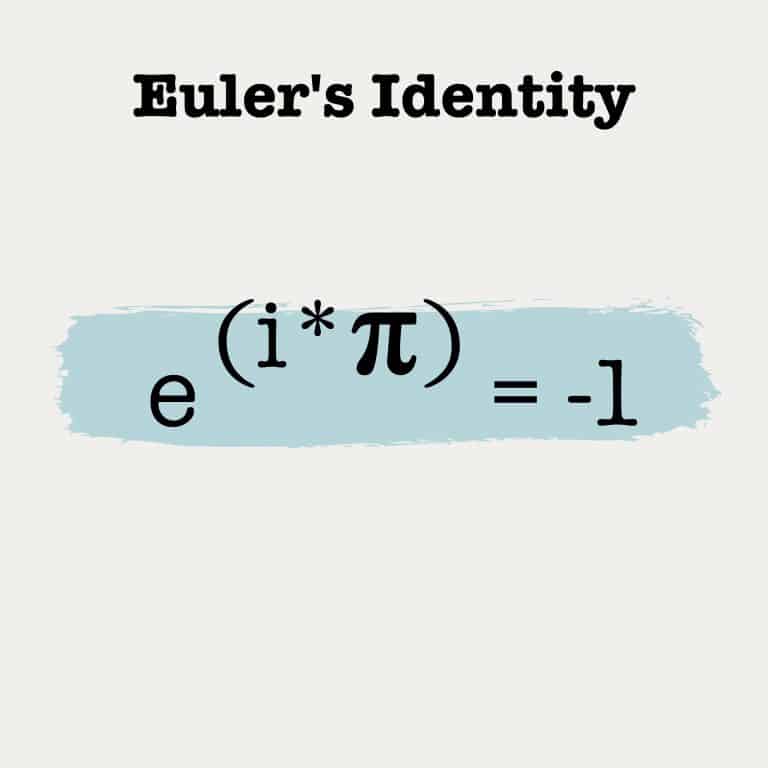
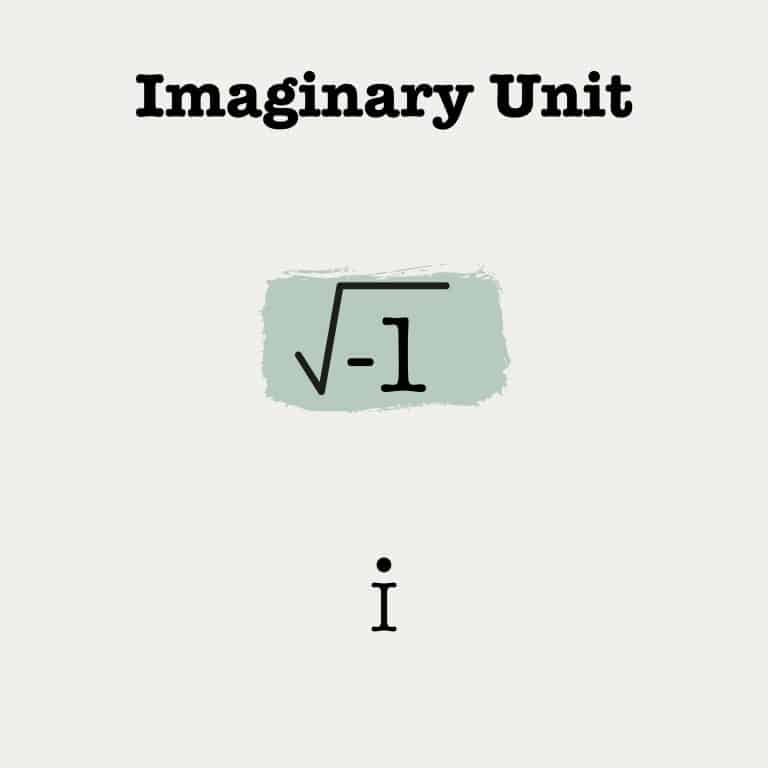The Avogadro constant is a number used to explain atoms, molecules, ions, and electrons. It is named after the Italian scientist Amedeo Avogadro and is defined exactly as NA = 6.02214076×1023 mol−1. For elements, the relative atomic mass expressed in grams contains the Avogadro Constant of atoms. For instance, a mole of Carbon has exactly 6.022 x 1023 atoms of carbon, and if you weigh it, it would weigh 12.011 grams, carbon’s atomic weight.
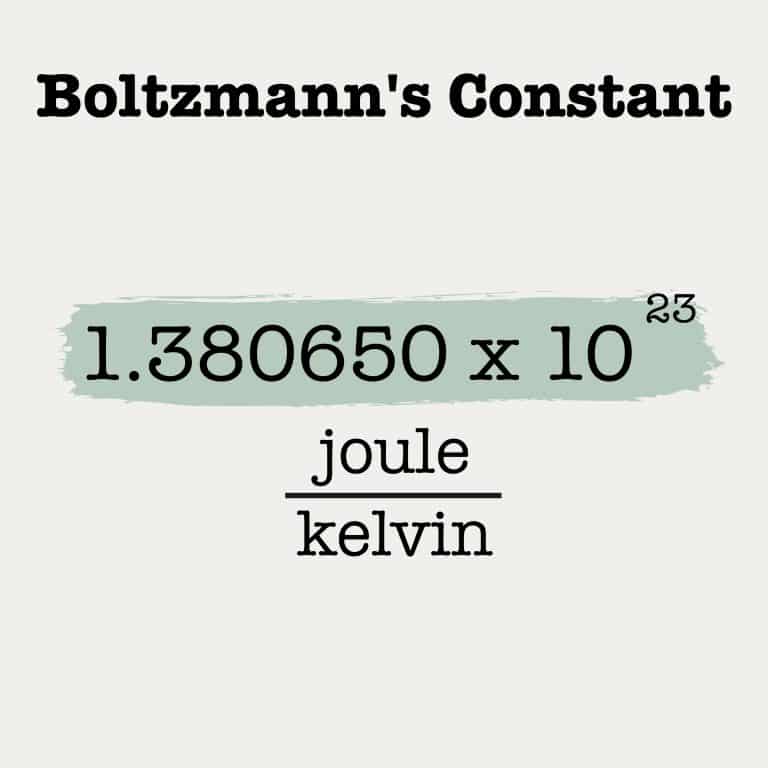
Boltzmann’s Constant
The fundamental constant of physics, the Boltzmann constant, is one of the seven "defining constants" that have been given exact definitions.